 We are a group of experimental and computational neuroscientists at the Janelia Research Campus of the Howard Hughes Medical Institute. We seek to understand how the brain gives rise to the rich, complex, and flexible behavioral patterns of animals. We believe that many fundamental principles underlying brain function have yet to be imagined and discovered, residing in the interactions across spatial and temporal scales between large neural circuits and molecular processes. Our goal is to connect molecular, cellular, and circuit phenomena to computational principles underlying behavior. We use molecular techniques, microscopy, engineering, and computational approaches to analyze the contribution of specific circuits and cell types to cognitive and behavioral processes.
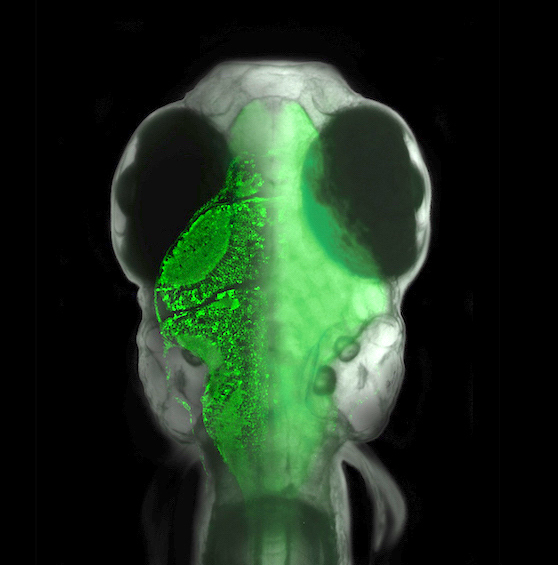
We are a group of experimental and computational neuroscientists at the Janelia Research Campus of the Howard Hughes Medical Institute. We seek to understand how the brain gives rise to the rich, complex, and flexible behavioral patterns of animals. We believe that many fundamental principles underlying brain function have yet to be imagined and discovered, residing in the interactions across spatial and temporal scales between large neural circuits and molecular processes. Our goal is to connect molecular, cellular, and circuit phenomena to computational principles underlying behavior. We use molecular techniques, microscopy, engineering, and computational approaches to analyze the contribution of specific circuits and cell types to cognitive and behavioral processes.Our favorite model organism is the larval zebrafish. This animal has an extensive behavioral repertoire even at one week of age, can be genetically manipulated, is transparent, and its brain is small enough to fit entirely under a microscope objective. That allows us to perform whole-brain imaging and optogenetic manipulation experiments in animals swimming through virtual environments, in search of the neural basis of complex behavior.
Find out more about us and our research through the examles and selected publications below, or come talk to us!
 Brain states, neuromodulation, and glia
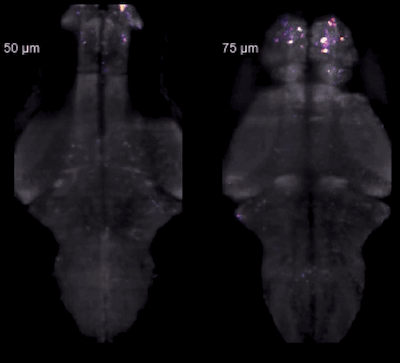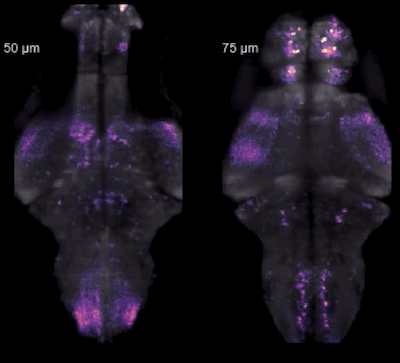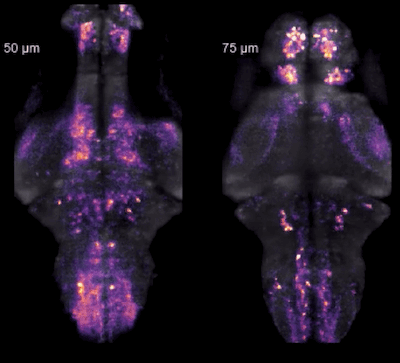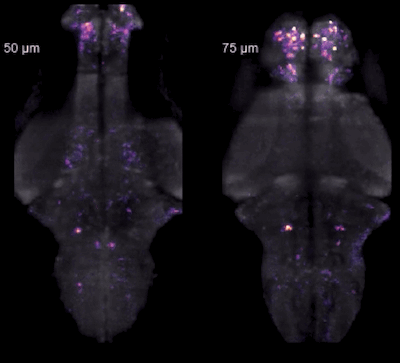
Brain states, neuromodulation, and gliaAnimals can shift between behavioral states, where they respond to sensory information and explore their environment in different ways. Some of these states arise because of accumulation of information about what happens when the animal performs certain actions. We study a behavior termed futility-induced passivity. When actions, like swimming, consistently fail to achieve their goals, like moving through the environment, animals tend to switch to a different behavior or even become passive for a period. In zebrafish this has been studied by placing animals in virtual arenas and switching from a closed-loop environment, where swimming leads to simulated displacement, to an open-loop environment, where swimming is futile and no longer affects the visual environment. By imaging from both neurons and a glial cell type termed radial astrocytes, it was found that noradrenergic neurons detect swim failures - i.e., swim bouts that do not lead to displacement. Such noradrenergic failure signals were integrated over periods of many seconds by radial astrocytes. Through glia-neuron interactions, the astrocytes then triggered a passive behavioral state that lasted many seconds, in part by the activation of nearby GABAergic neurons that suppress swimming. This work showed that astrocytes can play specific roles in neural computation, and can cause shifts in behavioral state. Relevant publication: Mu et al., Cell 2019
Related work centers on motor learning and its relation to neuromodulation. Important persistent changes in motor patterns can arise when animals need to change the vigor with which they move in response to changes in their environment or their body. The neural drive to the muscles to achieve a certain outcome can vary over time, e.g. when environmental temperature drops muscles can become less effective (especially in cold-blooded animals); the same can happen if an animal becomes energy depleted. To tune the vigor of the brain’s motor outflow, brains have to keep track of the average effectiveness of their actions over time, store this, and use it to adjust future motor vigor. This phenomenon has been studied in zebrafish in the context of swimming, where the feedback gain between motor vigor and visual flow was perturbed to induce compensatory changes in motor vigor. Whole-brain light-sheet imaging showed that visual feedback during swimming, indicative of how far the animal traveled as a result of a motor command, triggers activity in the serotonergic raphe nucleus that encodes this distance - a measure of the efficacy of swim commands. This activity builds up and persists over time, and causes a reduction of motor vigor, allowing the animal to adapt to changes in feedback gain: high feedback gain causes high levels of persistent raphe activity, which tunes down motor vigor and normalizes distance traveled per motor command. Whole-brain imaging during behavior was essential for finding the neural substrate for this persistent state. We published these findings in Kawashima et al., Cell 2016
 Technology develompent: whole-brain imaging, voltage imaging, optogenetics, computation
Technology develompent: whole-brain imaging, voltage imaging, optogenetics, computationTo study interactions between behavior, neurons and glia across the entire brain, we develop new technology for studying, analyzing and manipulating neural activity at the whole-brain scale. Here are examples of our tehnical work:
Function-guided brain-wide neural perturbation.
We combined whole-brain imaging with optogenetic perturbation and single-cell ablation. This technique allows for whole-brain functional mapping, followed by cell-resolution perturbation. Combined with computational analysis, it enables "on the fly hypothesis testing" -- measure activity everywhere; build a model; perturb brain activity to test the model.
Vladimirov et al., Nature Methods 2018
Distributed computation for analysis of large-scale data.
Whole-brain imaging generates large datasets, often hundreds of gigabytes per experiments. These datasets are hard to analyze on single workstations, and frequently require computer clusters. In this paper, we developed computational infrastructure for analyzing such datasets with distributed computation. Example analyses here include dimensionality reduction, whole-brain direction tuning, and others.
Freeman et al., Nature Methods 2016
Whole-brain imaging during virtual-reality behavior.
To perform whole-brain imaging in behaving zebrafish, we developed a system that combines light-sheet imaging with a virtual-reality system. Larval zebrafish can "swim around" in 2D virtual reality environments through a system that reads off their intended locomotion from electrical EEG activity from the tail motor nerves. A dual light-sheet microscope records whole-brain activity. We were very inspired by previous work in fruit flies rodents that similarly used VR environments to study neural activity in behaving animals.
Vladimirov et al., Nature Methods 2014
Whole-brain light-sheet imaging in zebrafish.
A "holy grail" in systems neuroscience has been to record from all neurons in the brain of a behaving animal. Here we did this using light-sheet imaging in zebrafish - an ideal combination of a technique and a suitable model organism: transparent, small, and genetically engineerable. Once you get whole-brain data, however, you realize what else you're missing! For example, calcium imaging is a slower and indirect readout of spiking. So we're not done yet!
Ahrens et al., Nature Methods 2013
Voltage imaging in zebrafish.
To get to spike-timing resolution, and image subthreshold voltage, we helped test voltage indicators in larval zebrafish. They work, currently in subpopulations of up to a few 100 cells. In the future we hope to scale this up to large brain volumes.
Abdelfattah et al., Science 2019
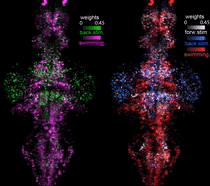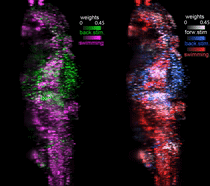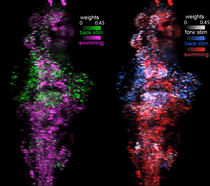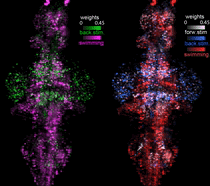
 Neural basis of exploratory behavior
Neural basis of exploratory behaviorIn the absence of sensory input, animals still need to explore their environments based on internal neural and bodily cues. How does intrinsic brain activity generate meaningful spontaneous behavior? How do such spontaneous networks interface with sensory-driven and goal-directed behavioral challenges - are these disjoint, or are spontaneously active circuits recruited to implement successively more complex behaviors? We are interested in such questions. Past example publications on this topic include
Dunn et al., eLife 2016
and on sensory-motor transformations:
Chen et al., Neuron 2018
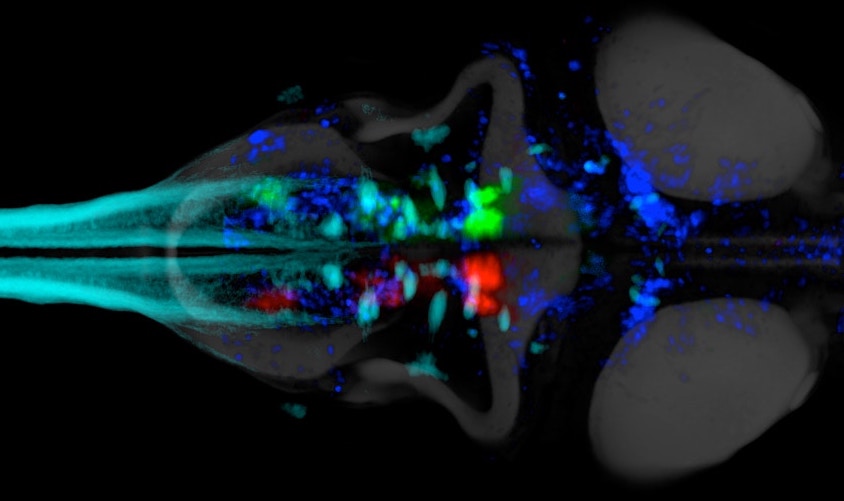
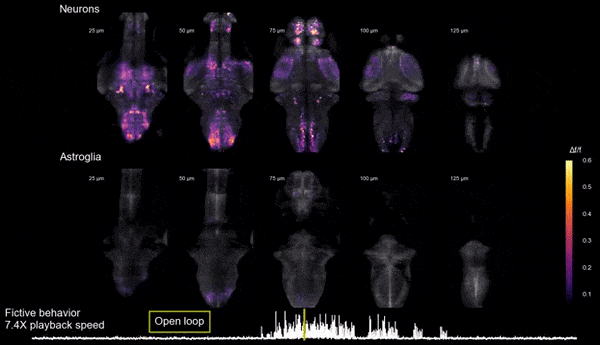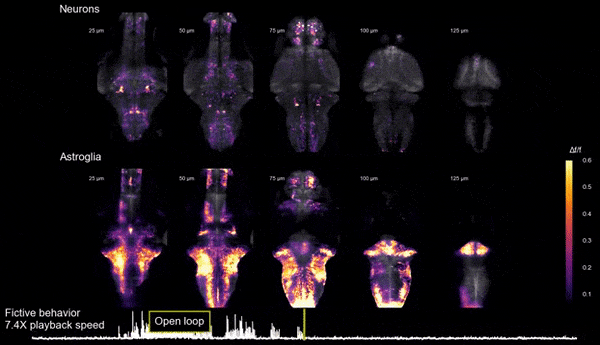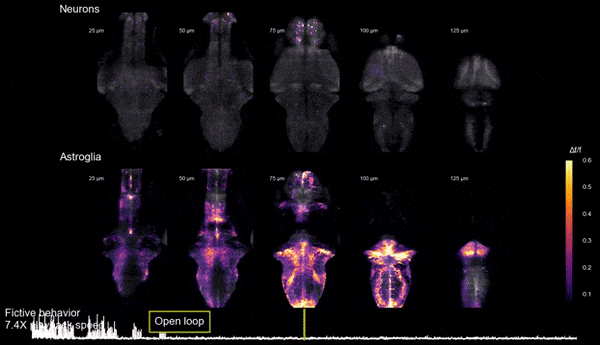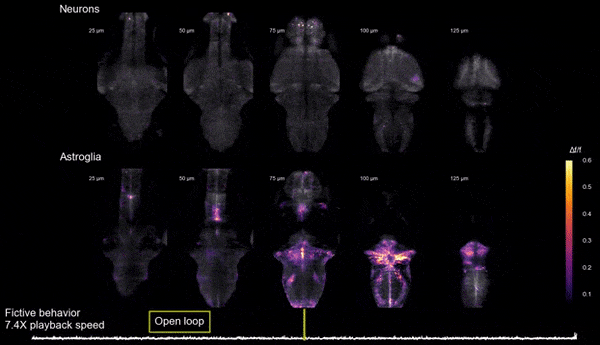 Glia-neuron interactions
Glia-neuron interactionsHaving discovered the circuit underlying futility-induced passivity (above), we can now use these cell types to delve into mechanisms of glia-neuron interactions - an area of biology with still many important open questions. How do these cell types 'talk to each other'? What molecules do they exchange? How are glia involved in neural computation? These are some of the questions we are exploring.
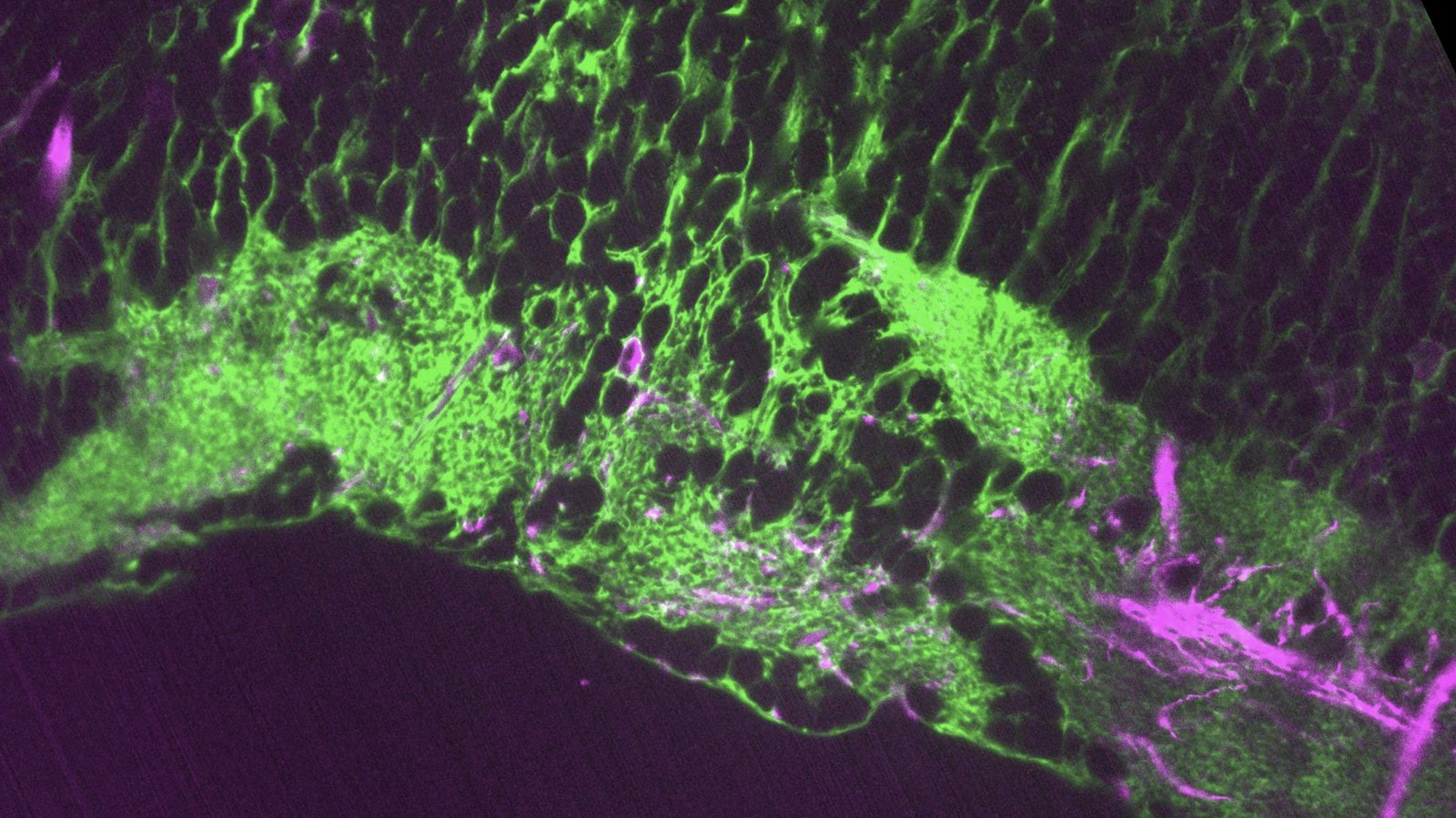 Further lab interests
Further lab interestsWe are also interested in the neural basis of learning and memory, and in technology development for neuroscience. And we are always open for collaborations.